I. Program Description
A. Overview
As stated on the Centers for Medicare & Medicaid Services (CMS) Medicaid site: The Medicaid Drug Rebate Program (MDRP) is a program that includes CMS, state Medicaid agencies, and participating drug manufacturers that helps to offset the Federal and state costs of most outpatient prescription drugs dispensed to Medicaid patients. All fifty states and the District of Columbia cover prescription drugs under the MDRP, which is authorized by Section 1927 of the Social Security Act.
The MDRP is designed to offset overall costs of prescription drugs under the Medicaid Program by requiring drug manufacturer to enter into, and have in effect, a National Drug Rebate Agreement (NDRA) with the Secretary of the Department of Health and Human Services (HHS) in exchange for state Medicaid coverage of most of the manufacturer’s drugs.
Manufacturers are responsible for paying a rebate on those drugs for which payment was made under the state plan. These rebates are paid by drug manufacturers on a quarterly basis to states and are shared between the states and the Federal government to offset the overall cost of prescription drugs under the Medicaid Program.
In addition to signing an NDRA, drug manufacturers are required to enter into agreements with two other Federal programs in order to have their drugs covered under Medicaid: a pricing agreement for the Section 340B Drug Pricing Program, administered by the Health Resources and Services Administration, and a master agreement with the Secretary of Veterans Affairs for the Federal Supply Schedule. Guam Medicaid currently has two (2) Federally Qualified Health Centers (FQHC)
that participate under the Section 340B Drug Pricing Program: The Northern Regional Health Center (NRHC) and The Southern Regional Health Center (SRHC). The medications dispensed by these two
providers are not eligible for the rebate since they in essence have already been discounted under the manufacturer pricing agreement for the Section 340B Drug Pricing Program mentioned above.
On February 1, 2016, the Centers for Medicare & Medicaid Services (CMS) published the “Medicaid Program; Covered Outpatient Drug” Final Rule with Comment Period (CMS-2345-FC) in the Federal Register (81 FR 5170). As part of that final rule with comment period, CMS amended the regulatory definitions of “States” and “United States” to include the U.S. Territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) beginning April 1, 2017. Inclusion of the territories in the definitions of “States” and “United States” allows Territories to participate in the Medicaid Drug Rebate Program (MDRP). Additionally, we indicated in the “Covered Outpatient Drug” final rule that territories are able to use existing waiver authority under Title XIX of the Social Security Act to elect not to participate in the MDRP, consistent with statutory provisions (81 FR 5170, 5204).
On November 15, 2016, CMS published an interim final rule with comment period that amended the regulatory definitions of “States” and “United States” to include the U.S. territories beginning April 1, 2020, rather than April 1, 2017 (interim final rule). However, on November 21, 2019, CMS issued “Medicaid Program; Covered Outpatient Drug; Further Delay of Inclusion of Territories in the Definitions of States and united States” Interim Final Rule with comment period that further delayed the inclusion of the U.S. territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) in the definitions of “States” and “United States” from April 1, 2020 until April 1, 2022. Then on November 19, 2021, the inclusion was delayed mainly due to the public health emergency until January 1, 2023. Because of the inclusion of territories in the definition of States and United States, Guam will be required to participate in the MDRP effective January 1, 2023. However, Guam is allowed to use the 1115 waiver authority to elect not to participate in the MDRP.
In light of the statutory MDRP directive, Guam Medicaid is seeking a waiver exempting them from the requirement to participate in the drug rebate program. Guam is requesting that the exemption from participating in the MDRP be effective from January 1, 2023 - December 31, 2027.
B. Summary of 1115 Waiver Demonstration Application Request
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
Historically, the Guam Medicaid funding has been limited under the Section 1108 annual block grant, and further limited by the established 55-45% FMAP. These limitations created an environment where the Guam Medicaid Program experienced hesitancy on the part of health care providers on island in participating in the program. The funding increases provided by the Patient Protection and Affordable Care Act (Obamacare) provided a temporary increase in funding, and recent legislation has further provided additional temporary funding and FMAP increases. These increases have helped the Guam Medicaid Program expand services and encouraged provider participation resulting in better recipient access to services that exist today.
Although the Medicaid Drug Rebate Program (MDRP) will offset costs of prescription drugs under the Guam Medicaid Program, the Territory has identified potential negative impacts for pharmacy providers which may ultimately affect the program’s ability to maintain its existing provider network
of on-island pharmacies essential for program participants to have adequate access to pharmacy services, and create added labor intensive program costs that may outweigh the benefits of participation in the MDRP.
The island Medicaid pharmacy providers often face challenges in obtaining supplies because of the remoteness of Guam’s location relative to supply chains. This often times creates challenges in the form of accepting higher wholesale pricing from distributors when purchasing pharmaceutical drugs, and paying for higher shipping costs because of the need to transport these supplies via air freight due to their inability to stockpile medication when considering drug expiration dates relative to expected sales and supply needs which often causes inventory or availability issues. This often times translates to more expensive costs that is requested as reimbursement for the sale of these medications to program recipients.
Additionally, due to the relatively insignificant total purchase amounts made through pharmaceutical distributors in comparison to the larger pharmacy chains in the mainland U.S., the island pharmacy providers are unable to negotiate for best prices or favorable shipping terms in obtaining medication supplies. Guam’s participation in the MDRP would place added pressures on the island Medicaid pharmacy providers because it would require them to carry all covered outpatient drugs (COD) of a participating manufacturer, and for the program to cover these CODs under Medicaid. Currently, almost all drug manufacturers are participating in the MDRP.
Guam Medicaid has managed to control the costs of Pharmacy expenditures because it currently controls their drug formulary which list covered medication under the program. However, participation in the MDRP would require that we cover all drugs of participating manufacturers, and essentially cover all COD drugs if the waiver application is not approved. This would be a substantial cost increase to Guam Medicaid. The current drug pricing for the program’s drug formulary is set at the Lowest Wholesale Price (LWP) listed on Redbook at the time the formulary is released in January of each calendar year. The program feels that during this waiver demonstration, they would be able to maintain substantial cost savings for their pharmacy expenditures by maintaining a relatively low expenditure rate for its pharmacy expense relative to total expenditures, and the approval of the 1115 waiver will allow the program a period of time to assess this standard in comparison with other state Medicaid programs that receive rebates under the MDRP.
Under this MDRP 1115 Waiver Demonstration application, the program will be able to continue to maintain control of its drug formulary and the covered outpatient drug (COD) coverage, and allow more time for the program to properly assess island pharmacy impacts, which is important because of potential drug inventory issues faced by the on-island pharmacies as previously mentioned due to our remoteness relative to mainland pharmaceutical supply lines. This waiver may prove to be more of a cost savings for Guam Medicaid when compared to potentially having to cover all drugs of manufacturers that enter into a rebate under the MDRP and create additional administrative burdens and costs due to additional labor-intensive costs for implementation activities associated with the implementation and participation in the MDRP.
C. Eligibility Requirements
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will affect the on-island pharmacy provider participation on the program which will affect adequate access for the program participants described in the chart below.
D. Health Care Delivery System and Benefits
This MDRP 1115 Waiver Demonstration Application is seeking to waive the programs participation in the MDRP and does not propose any changes to the Medicaid health care delivery system; Guam Medicaid enrollees will continue to receive services through the Territory’s fee-for-service delivery system. MAPNEG Program enrollees will also continue to receive benefits through the Alternative Benefit Plan; the Territory does not propose any changes to benefits for any program enrollees.
E. Cost Sharing
Cost sharing will not be affected under this 1115 Waiver Demonstration request.
II. Goals and Objectives
The overall goal of the demonstration is to assure the network capacity of on-island pharmacy providers remains consistent with the existing capacity prior to an implementation mandated for program participation in the MDRP in order to provide adequate recipient access to pharmacy services, and to allow time to properly assess potential adverse effects of participation in the MDRP on our island pharmacy providers, program recipients, and to assess additional administrative program costs related to the management of the MDRP that would possibly outweigh any rebate savings.
NOTE: The demonstration applies to all pharmacy services for authorized providers under the Guam Medicaid State Plan that dispense covered outpatient drugs (COD).
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP) than the rebate savings it provides, and may potentially impact provider (pharmacy) participation.
III. Enrollment Projections and Annual Expenditures
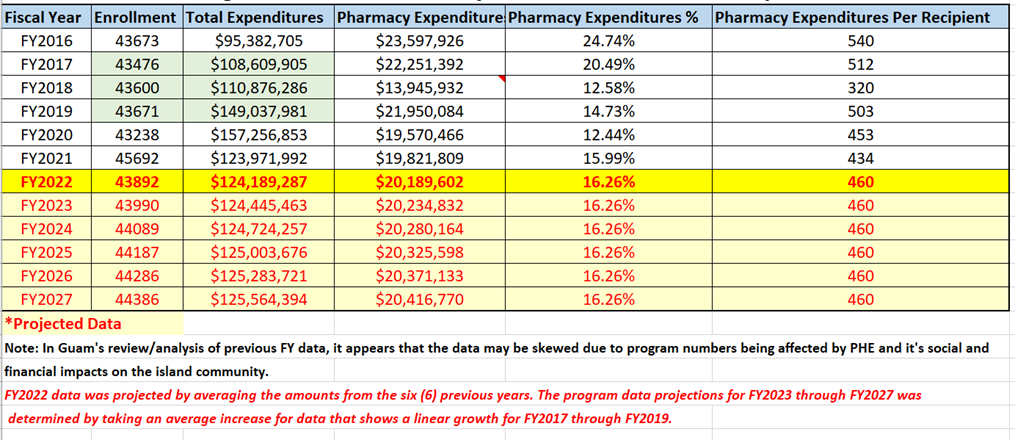
Guam Medicaid program’s historical enrollment figures for fiscal years 2016 to present and corresponding program year total program expenditures and pharmacy expenditures with projections for FY2022 through FY2027.
Figure 1. Guam Medicaid Program Historical and Projected - Enrollment and Expenditures Data
IV. Waiver Expenditure Authorities
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
V. Demonstration Hypotheses and Evaluation Parameters
Hypothesis: Guam Medicaid’s participation in the MDRP would adversely affect the On-Island Pharmacies by creating an unreasonable requirement for them to carry all of a participating manufacturer’s COD such that it would be difficult for them to maintain adequate inventory, and to continue as providers on the Medicaid program. Additionally, it would be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP), and these costs would outweigh the rebate savings MDRP would provide.
Qualitative methods will be employed to evaluate:
* The possible adverse effects to On-Island Pharmacy Providers should Guam participate in the MDRP;
* Current limitations for On-Island Pharmacies with existing supply chains when ordering CODs for dispensing to Medicaid recipients; and
* How Guam Medicaid’s Participation on the MDRP would affect pharmacy provider status on the Medicaid program.
Pharmacy surveys will be used for qualitative/quantitative methods.
Quantitative methods will be used to evaluate MDRP rebate savings relative to average program savings nationwide, and the following anticipated program costs for implementation of the MDRP (42 CFR 447.511).
* Cost Benefit Analysis to evaluate potential cost savings provided on the MDRP in relation to potential increases in administrative costs to the program due to requirements for contractual services to administer the tracking and reporting of the data for NDCs to the participating drug manufacturers to include an additional State Agency FTE position to be responsible for the MDRP requirements as a whole.
* Guam’s assessment of the following Costs associated with participation in the MDRP
* Guam’s assessment of costs for Integrating Physician Administered drugs into rebate processes (42 CFR 447.520)
Cost associated with invoicing all outpatient drugs administered in a clinical setting or emergency department. Provider training regarding HCPCS coding system in these settings for claims to reference National Drug Code (NDC) or assess costs to perform a crosswalk from the HCPCS to the NDC. (Capture additional work and support needed to integrate these claims into the invoicing process for rebates).
* Guam’s assessment of the Costs associated with requiring Guam to use actual acquisition cost (AAC) + dispensing fee methodology (42 CFR 447.512)
Cost to pay AAC, if MDRP implemented, requiring Guam to survey its pharmacies regarding their acquisition costs in order to determine if using AWP is close to the pharmacies’ acquisition costs and therefore can be used as a basis for reimbursement. A survey would also need to be done to ascertain dispensing fee costs of your pharmacies. (Estimates: Possible cost of $50,000 -100,000 for the acquisition cost survey and another $50,000-100,000 to do cost of dispensing fee as needed. (RFQs to assess survey costs).
* Assessment of the Drug Utilization Review (DUR) – 1927(g) and 42 CFR 456.703
1. RFQ to assess costs to contract a Provider Benefits Manager (PBM), to conduct Prospective Drug Utilization Review processes (PRO-DUR). All PBMs offer PRO-DUR as part of the claims processing, but need to ascertain any additional costs related to those services.
2. Assess feasibility to work with UPIC West, Qlarant in conducting required Retrospective Drug Utilization Reviews (RETRO-DUR). Guam currently working with CMS contractor as part of program integrity reviews to fight fraud, waste and abuse. (Assess any additional cost).
* Assessment and cost breakdown of FTE staff required to be responsible for performing duties related to the Medicaid drug rebate system (Medicaid Drug Product, or MDP) Assess cost associated for a state employee at a minimum of 1/2 of an FTE on Guam’s Medicaid staff. (Employee to act as point person for all activities regarding the Medicaid drug benefit to include rebate and Drug Utilization Review, and managing the MDRP contract).
VI. Public Review and Comment Process
The complete version of the application is available for public review at http://dphss.guam.gov/category/press-releases-en/.
Paper copies are available to be picked up in person at the Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
Two public meetings will be held regarding the Demonstration application:
(1) Public hearing on Monday, June 27, 2022 from 1:00 p.m. to 3:00 p.m. CHST (Guam Congress Building, Public Hearing Room, 163 Chalan Santo Papa, Hagåtña, Guam 96910). This hearing will be broadcast on GTA TV Channel 21, Docomo Channel 117/112.4, and livestream on https://www.youtube.com/c/GuamLegislatureMedia
(2) In-Person/Virtual (Zoom) Public hearing on Friday, July 8, 2022 from 1:00 p.m. to 3:00 p.m. CHST. DPHSS In-Person and Virtual (Zoom) Information Hearing. (Governor’s Conference Room, Ricardo J. Bordallo Complex, 513 West Marine Corps Drive, Hagatna, Guam 96910, 1:00 p.m. to 3:00 p.m. CHST). Public comments can be made by phone on the day of the hearing by calling the following number: (671) 473-1165.
Zoom Hearing Registration link can be obtained by emailing Jeffrey San Nicolas at Jeffrey.sannicolas@dphss.guam.gov or at the following site: http://dphss.guam.gov/category/press-releases-en/.
Public comments may be submitted until 11:59 PM (CHST) on July 24, 2022. Hard copy questions or public comments may be addressed to: Guam Medicaid – MDRP 1115 Waiver Demonstration, Department of Public Health & Social Services, BHCFA, 155 Hesler Place, Hagatna, Guam 96910, or by telephone to (671) 735-7470, or by electronic mail to michael.gallo@dphss.guam.gov. Please note that comments will continue to be accepted after August 1, 2022, but Guam Medicaid may not be able to consider those comments prior to the initial submission of the 1115 Waiver Demonstration application to CMS.
After Guam Medicaid reviews comments submitted during this public comment period, it will submit a revised application to CMS. Interested parties will also have an opportunity to officially comment during the 30-day federal public comment period; the submitted application will be available for comment on the CMS website at https://www.medicaid.gov/medicaid/section-1115-demo/demonstration-and-waiver-list/index.html.
Copies of this notice are available at the Government of Guam, Department of Public Health and Social Services website at http://dphss.guam.gov/category/press-releases-en/ for public review. Additional information concerning this action is available upon request at the address cited below.
Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
A. Overview
As stated on the Centers for Medicare & Medicaid Services (CMS) Medicaid site: The Medicaid Drug Rebate Program (MDRP) is a program that includes CMS, state Medicaid agencies, and participating drug manufacturers that helps to offset the Federal and state costs of most outpatient prescription drugs dispensed to Medicaid patients. All fifty states and the District of Columbia cover prescription drugs under the MDRP, which is authorized by Section 1927 of the Social Security Act.
The MDRP is designed to offset overall costs of prescription drugs under the Medicaid Program by requiring drug manufacturer to enter into, and have in effect, a National Drug Rebate Agreement (NDRA) with the Secretary of the Department of Health and Human Services (HHS) in exchange for state Medicaid coverage of most of the manufacturer’s drugs.
Manufacturers are responsible for paying a rebate on those drugs for which payment was made under the state plan. These rebates are paid by drug manufacturers on a quarterly basis to states and are shared between the states and the Federal government to offset the overall cost of prescription drugs under the Medicaid Program.
In addition to signing an NDRA, drug manufacturers are required to enter into agreements with two other Federal programs in order to have their drugs covered under Medicaid: a pricing agreement for the Section 340B Drug Pricing Program, administered by the Health Resources and Services Administration, and a master agreement with the Secretary of Veterans Affairs for the Federal Supply Schedule. Guam Medicaid currently has two (2) Federally Qualified Health Centers (FQHC)
that participate under the Section 340B Drug Pricing Program: The Northern Regional Health Center (NRHC) and The Southern Regional Health Center (SRHC). The medications dispensed by these two
providers are not eligible for the rebate since they in essence have already been discounted under the manufacturer pricing agreement for the Section 340B Drug Pricing Program mentioned above.
On February 1, 2016, the Centers for Medicare & Medicaid Services (CMS) published the “Medicaid Program; Covered Outpatient Drug” Final Rule with Comment Period (CMS-2345-FC) in the Federal Register (81 FR 5170). As part of that final rule with comment period, CMS amended the regulatory definitions of “States” and “United States” to include the U.S. Territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) beginning April 1, 2017. Inclusion of the territories in the definitions of “States” and “United States” allows Territories to participate in the Medicaid Drug Rebate Program (MDRP). Additionally, we indicated in the “Covered Outpatient Drug” final rule that territories are able to use existing waiver authority under Title XIX of the Social Security Act to elect not to participate in the MDRP, consistent with statutory provisions (81 FR 5170, 5204).
On November 15, 2016, CMS published an interim final rule with comment period that amended the regulatory definitions of “States” and “United States” to include the U.S. territories beginning April 1, 2020, rather than April 1, 2017 (interim final rule). However, on November 21, 2019, CMS issued “Medicaid Program; Covered Outpatient Drug; Further Delay of Inclusion of Territories in the Definitions of States and united States” Interim Final Rule with comment period that further delayed the inclusion of the U.S. territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) in the definitions of “States” and “United States” from April 1, 2020 until April 1, 2022. Then on November 19, 2021, the inclusion was delayed mainly due to the public health emergency until January 1, 2023. Because of the inclusion of territories in the definition of States and United States, Guam will be required to participate in the MDRP effective January 1, 2023. However, Guam is allowed to use the 1115 waiver authority to elect not to participate in the MDRP.
In light of the statutory MDRP directive, Guam Medicaid is seeking a waiver exempting them from the requirement to participate in the drug rebate program. Guam is requesting that the exemption from participating in the MDRP be effective from January 1, 2023 - December 31, 2027.
B. Summary of 1115 Waiver Demonstration Application Request
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
Historically, the Guam Medicaid funding has been limited under the Section 1108 annual block grant, and further limited by the established 55-45% FMAP. These limitations created an environment where the Guam Medicaid Program experienced hesitancy on the part of health care providers on island in participating in the program. The funding increases provided by the Patient Protection and Affordable Care Act (Obamacare) provided a temporary increase in funding, and recent legislation has further provided additional temporary funding and FMAP increases. These increases have helped the Guam Medicaid Program expand services and encouraged provider participation resulting in better recipient access to services that exist today.
Although the Medicaid Drug Rebate Program (MDRP) will offset costs of prescription drugs under the Guam Medicaid Program, the Territory has identified potential negative impacts for pharmacy providers which may ultimately affect the program’s ability to maintain its existing provider network
of on-island pharmacies essential for program participants to have adequate access to pharmacy services, and create added labor intensive program costs that may outweigh the benefits of participation in the MDRP.
The island Medicaid pharmacy providers often face challenges in obtaining supplies because of the remoteness of Guam’s location relative to supply chains. This often times creates challenges in the form of accepting higher wholesale pricing from distributors when purchasing pharmaceutical drugs, and paying for higher shipping costs because of the need to transport these supplies via air freight due to their inability to stockpile medication when considering drug expiration dates relative to expected sales and supply needs which often causes inventory or availability issues. This often times translates to more expensive costs that is requested as reimbursement for the sale of these medications to program recipients.
Additionally, due to the relatively insignificant total purchase amounts made through pharmaceutical distributors in comparison to the larger pharmacy chains in the mainland U.S., the island pharmacy providers are unable to negotiate for best prices or favorable shipping terms in obtaining medication supplies. Guam’s participation in the MDRP would place added pressures on the island Medicaid pharmacy providers because it would require them to carry all covered outpatient drugs (COD) of a participating manufacturer, and for the program to cover these CODs under Medicaid. Currently, almost all drug manufacturers are participating in the MDRP.
Guam Medicaid has managed to control the costs of Pharmacy expenditures because it currently controls their drug formulary which list covered medication under the program. However, participation in the MDRP would require that we cover all drugs of participating manufacturers, and essentially cover all COD drugs if the waiver application is not approved. This would be a substantial cost increase to Guam Medicaid. The current drug pricing for the program’s drug formulary is set at the Lowest Wholesale Price (LWP) listed on Redbook at the time the formulary is released in January of each calendar year. The program feels that during this waiver demonstration, they would be able to maintain substantial cost savings for their pharmacy expenditures by maintaining a relatively low expenditure rate for its pharmacy expense relative to total expenditures, and the approval of the 1115 waiver will allow the program a period of time to assess this standard in comparison with other state Medicaid programs that receive rebates under the MDRP.
Under this MDRP 1115 Waiver Demonstration application, the program will be able to continue to maintain control of its drug formulary and the covered outpatient drug (COD) coverage, and allow more time for the program to properly assess island pharmacy impacts, which is important because of potential drug inventory issues faced by the on-island pharmacies as previously mentioned due to our remoteness relative to mainland pharmaceutical supply lines. This waiver may prove to be more of a cost savings for Guam Medicaid when compared to potentially having to cover all drugs of manufacturers that enter into a rebate under the MDRP and create additional administrative burdens and costs due to additional labor-intensive costs for implementation activities associated with the implementation and participation in the MDRP.
C. Eligibility Requirements
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will affect the on-island pharmacy provider participation on the program which will affect adequate access for the program participants described in the chart below.
| Eligibility Group Name | Social Security Act and CFR Citations | Income Level |
| Guam Medicaid Program New Adult Group (MAPNEG), Elderly and Disabled |
Social Security Act 1396(a)(10)(A)(i)(VIII) 42 C.F.R. 435.119 |
New adult group, Elderly and Disabled group with income 0-138 percent LPL |
D. Health Care Delivery System and Benefits
This MDRP 1115 Waiver Demonstration Application is seeking to waive the programs participation in the MDRP and does not propose any changes to the Medicaid health care delivery system; Guam Medicaid enrollees will continue to receive services through the Territory’s fee-for-service delivery system. MAPNEG Program enrollees will also continue to receive benefits through the Alternative Benefit Plan; the Territory does not propose any changes to benefits for any program enrollees.
E. Cost Sharing
Cost sharing will not be affected under this 1115 Waiver Demonstration request.
II. Goals and Objectives
The overall goal of the demonstration is to assure the network capacity of on-island pharmacy providers remains consistent with the existing capacity prior to an implementation mandated for program participation in the MDRP in order to provide adequate recipient access to pharmacy services, and to allow time to properly assess potential adverse effects of participation in the MDRP on our island pharmacy providers, program recipients, and to assess additional administrative program costs related to the management of the MDRP that would possibly outweigh any rebate savings.
NOTE: The demonstration applies to all pharmacy services for authorized providers under the Guam Medicaid State Plan that dispense covered outpatient drugs (COD).
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP) than the rebate savings it provides, and may potentially impact provider (pharmacy) participation.
III. Enrollment Projections and Annual Expenditures
Guam Medicaid program’s historical enrollment figures for fiscal years 2016 to present and corresponding program year total program expenditures and pharmacy expenditures with projections for FY2022 through FY2027.
Figure 1. Guam Medicaid Program Historical and Projected - Enrollment and Expenditures Data

IV. Waiver Expenditure Authorities
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
V. Demonstration Hypotheses and Evaluation Parameters
Hypothesis: Guam Medicaid’s participation in the MDRP would adversely affect the On-Island Pharmacies by creating an unreasonable requirement for them to carry all of a participating manufacturer’s COD such that it would be difficult for them to maintain adequate inventory, and to continue as providers on the Medicaid program. Additionally, it would be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP), and these costs would outweigh the rebate savings MDRP would provide.
Qualitative methods will be employed to evaluate:
* The possible adverse effects to On-Island Pharmacy Providers should Guam participate in the MDRP;
* Current limitations for On-Island Pharmacies with existing supply chains when ordering CODs for dispensing to Medicaid recipients; and
* How Guam Medicaid’s Participation on the MDRP would affect pharmacy provider status on the Medicaid program.
Pharmacy surveys will be used for qualitative/quantitative methods.
Quantitative methods will be used to evaluate MDRP rebate savings relative to average program savings nationwide, and the following anticipated program costs for implementation of the MDRP (42 CFR 447.511).
* Cost Benefit Analysis to evaluate potential cost savings provided on the MDRP in relation to potential increases in administrative costs to the program due to requirements for contractual services to administer the tracking and reporting of the data for NDCs to the participating drug manufacturers to include an additional State Agency FTE position to be responsible for the MDRP requirements as a whole.
* Guam’s assessment of the following Costs associated with participation in the MDRP
- Cost of contractor to process claims electronically and invoice manufacturer rebates. (Review of small fee-for-service state, to determine costs for claims processing and invoicing via contractor (e.g., Magellan or Change Healthcare Vendors for minimum cost required to invoice manufacturers, run dispute resolution, and collect money etc.).
- Costs involved in developing MDRP Participating Manufacturers NDC Drug Formulary Listing and evaluation of feasibility of developing procedures to prior authorize (PA) drugs to ensure the drugs are from participating manufacturers. (Assess cost and additional labor involved in this procedure).
* Guam’s assessment of costs for Integrating Physician Administered drugs into rebate processes (42 CFR 447.520)
Cost associated with invoicing all outpatient drugs administered in a clinical setting or emergency department. Provider training regarding HCPCS coding system in these settings for claims to reference National Drug Code (NDC) or assess costs to perform a crosswalk from the HCPCS to the NDC. (Capture additional work and support needed to integrate these claims into the invoicing process for rebates).
* Guam’s assessment of the Costs associated with requiring Guam to use actual acquisition cost (AAC) + dispensing fee methodology (42 CFR 447.512)
Cost to pay AAC, if MDRP implemented, requiring Guam to survey its pharmacies regarding their acquisition costs in order to determine if using AWP is close to the pharmacies’ acquisition costs and therefore can be used as a basis for reimbursement. A survey would also need to be done to ascertain dispensing fee costs of your pharmacies. (Estimates: Possible cost of $50,000 -100,000 for the acquisition cost survey and another $50,000-100,000 to do cost of dispensing fee as needed. (RFQs to assess survey costs).
* Assessment of the Drug Utilization Review (DUR) – 1927(g) and 42 CFR 456.703
1. RFQ to assess costs to contract a Provider Benefits Manager (PBM), to conduct Prospective Drug Utilization Review processes (PRO-DUR). All PBMs offer PRO-DUR as part of the claims processing, but need to ascertain any additional costs related to those services.
2. Assess feasibility to work with UPIC West, Qlarant in conducting required Retrospective Drug Utilization Reviews (RETRO-DUR). Guam currently working with CMS contractor as part of program integrity reviews to fight fraud, waste and abuse. (Assess any additional cost).
* Assessment and cost breakdown of FTE staff required to be responsible for performing duties related to the Medicaid drug rebate system (Medicaid Drug Product, or MDP) Assess cost associated for a state employee at a minimum of 1/2 of an FTE on Guam’s Medicaid staff. (Employee to act as point person for all activities regarding the Medicaid drug benefit to include rebate and Drug Utilization Review, and managing the MDRP contract).
VI. Public Review and Comment Process
The complete version of the application is available for public review at http://dphss.guam.gov/category/press-releases-en/.
Paper copies are available to be picked up in person at the Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
Two public meetings will be held regarding the Demonstration application:
(1) Public hearing on Monday, June 27, 2022 from 1:00 p.m. to 3:00 p.m. CHST (Guam Congress Building, Public Hearing Room, 163 Chalan Santo Papa, Hagåtña, Guam 96910). This hearing will be broadcast on GTA TV Channel 21, Docomo Channel 117/112.4, and livestream on https://www.youtube.com/c/GuamLegislatureMedia
(2) In-Person/Virtual (Zoom) Public hearing on Friday, July 8, 2022 from 1:00 p.m. to 3:00 p.m. CHST. DPHSS In-Person and Virtual (Zoom) Information Hearing. (Governor’s Conference Room, Ricardo J. Bordallo Complex, 513 West Marine Corps Drive, Hagatna, Guam 96910, 1:00 p.m. to 3:00 p.m. CHST). Public comments can be made by phone on the day of the hearing by calling the following number: (671) 473-1165.
Zoom Hearing Registration link can be obtained by emailing Jeffrey San Nicolas at Jeffrey.sannicolas@dphss.guam.gov or at the following site: http://dphss.guam.gov/category/press-releases-en/.
Public comments may be submitted until 11:59 PM (CHST) on July 24, 2022. Hard copy questions or public comments may be addressed to: Guam Medicaid – MDRP 1115 Waiver Demonstration, Department of Public Health & Social Services, BHCFA, 155 Hesler Place, Hagatna, Guam 96910, or by telephone to (671) 735-7470, or by electronic mail to michael.gallo@dphss.guam.gov. Please note that comments will continue to be accepted after August 1, 2022, but Guam Medicaid may not be able to consider those comments prior to the initial submission of the 1115 Waiver Demonstration application to CMS.
After Guam Medicaid reviews comments submitted during this public comment period, it will submit a revised application to CMS. Interested parties will also have an opportunity to officially comment during the 30-day federal public comment period; the submitted application will be available for comment on the CMS website at https://www.medicaid.gov/medicaid/section-1115-demo/demonstration-and-waiver-list/index.html.
Copies of this notice are available at the Government of Guam, Department of Public Health and Social Services website at http://dphss.guam.gov/category/press-releases-en/ for public review. Additional information concerning this action is available upon request at the address cited below.
Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
Please click on the link below to view a PDF copy of the DRAFT Application:








